In the rapidly evolving world of advanced ceramics and functional materials, nitrides have emerged as essential compounds for high-performance applications. Whether in semiconductors, high-temperature structural ceramics, or protective coatings, understanding nitrides begins with a fundamental concept: the nitride symbol.

The nitride symbol is more than a simple notation; it represents chemical composition, valency, charge, and bonding behavior. For engineers, researchers, and factory procurement professionals, mastery of this symbol and its associated compounds is vital to selecting the right materials for applications requiring thermal stability, electrical conductivity, or mechanical robustness.
This article will explore nitrides comprehensively, including:
-
Fundamentals of nitrides and their chemical symbols
-
Classification and structure
-
Core nitride compounds: GaN, AlN, Si₃N₄, TiN, CrN, and c-BN
-
Chemical reactions of nitrides, including sodium nitride, calcium nitride, water interactions, and hydrogen absorption
-
Industrial applications
-
FAQs and hot topics regarding nitride symbols and compounds
By the end of this guide, readers will understand how the chemical symbol for nitride, Lewis symbol for nitride ion, and nitride symbol and valency connect to real-world performance.
Nitride Basics
What is a Nitride?
A nitride is a chemical compound in which nitrogen combines with a less electronegative element, often a metal or metalloid. Nitrogen typically exhibits a –3 oxidation state, forming the nitride ion symbol (N³⁻).
The Lewis symbol for the nitride ion is represented as:
..
: N :
..
This structure shows nitrogen with a filled octet, highlighting its ability to accept three electrons. The nitride symbol and charge is therefore N³⁻, reflecting both valency and ionic character.
Nitride Types
Understanding the Nitride Symbol
Nitride Ion Symbol and Valency
-
Nitride Ion Symbol: N³⁻
-
Valency: 3
-
Charge: –3
-
Lewis Symbol for Nitride Ion: :N:³⁻
These representations allow chemists and engineers to predict stoichiometry and bonding behavior in various compounds.
Chemical Symbol for Common Nitrides
| Compound | Symbol | Type | 참고 |
|---|---|---|---|
| 질화 알루미늄 | AlN | Covalent/Ionic | High thermal conductivity, insulating |
| Gallium Nitride | GaN | Covalent | Wide-bandgap semiconductor |
| 질화규소 | Si₃N₄ | Covalent | Structural ceramics |
| Titanium Nitride | TiN | transition metal nitrides | Hard coating, wear-resistant |
| Chromium Nitride | CrN | transition metal nitrides | Protective surface coatings |
| Cubic Boron Nitride | BN | Covalent | Ultra-hard material |
Core Nitride Compounds and Industrial Importance
Gallium Nitride (GaN) – The Semiconductor Workhorse
-
Symbol: GaN
-
Properties: Wide bandgap (~3.4 eV), high electron mobility, robust thermal and electrical performance.
-
Applications: LEDs, power electronics, RF amplifiers.
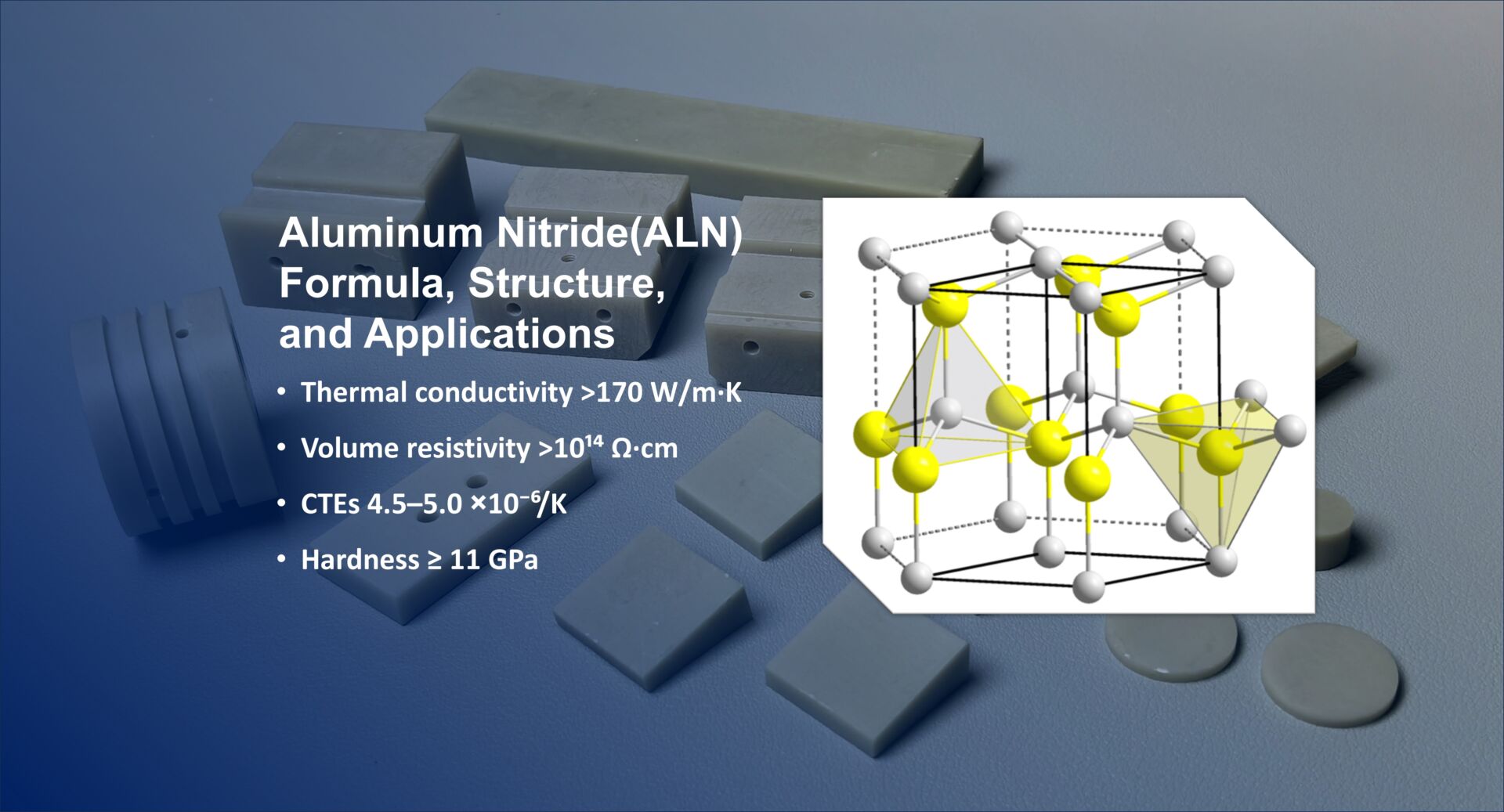
Aluminium Nitride (AlN) – Thermal Management Champion
-
Aluminium Nitride Symbol: AlN
-
Properties: High thermal conductivity (~285 W/m·K), electrical insulation, low thermal expansion.
-
Applications: Substrates for power electronics, heat spreaders, LED packaging.
Silicon Nitride (Si₃N₄) – Mechanical Reliability
-
Symbol: Si₃N₄
-
Properties: High fracture toughness, thermal shock resistance, chemical inertness.
-
Applications: Bearings, engine components, cutting tools.
Cubic Boron Nitride (c-BN) – Ultra-Hard Coatings
-
Symbol: BN
-
Properties: Second only to diamond in hardness, high thermal stability.
-
Applications: Cutting tools, abrasive coatings.
Titanium Nitride (TiN) and Chromium Nitride (CrN) – Protective Coatings
-
Symbols: TiN, CrN
-
Properties: Hard, wear-resistant, corrosion-resistant.
-
Applications: Tool coatings, aerospace components, decorative films.
Nitride Chemical Reactions
Understanding nitride reactivity is critical for processing, handling, and material design.
Sodium Nitride (Na₃N) Reactivity
-
Stability: Highly unstable, decomposes readily at room temperature.
-
Decomposition:
Na₃N → 3Na + ½ N₂ -
Reaction with Water:
Na₃N + 3H₂O → 3NaOH + NH₃↑
Formation of Calcium Nitride (Ca₃N₂)
-
Synthesis:
3Ca + N₂ → Ca₃N₂ (high temperature) -
Reactivity with Water:
Ca₃N₂ + 6H₂O → 3Ca(OH)₂ + 2NH₃↑ -
Notes: Common method for ammonia production and high-temperature ceramic precursors.
Interaction with Water
-
Alkali Metal Nitrides (Li₃N, Na₃N): Rapid hydrolysis releasing NH₃.
-
Alkaline Earth Nitrides (Ca₃N₂, Mg₃N₂): Moderate reaction, forms hydroxides and ammonia.
-
Covalent Nitrides (AlN, Si₃N₄): React slowly with water; generally stable under ambient conditions but can hydrolyze under acidic or basic conditions.
Hydrogen Absorption
Some transition metal nitrides (VN, TiN, NbN) can absorb hydrogen into their lattice:
MN + xH₂ ↔ MNHₓ
- Applications: Hydrogen storage, catalysis, high-temperature hydrogen-resistant materials.
Industrial Applications of Nitrides
자주 묻는 질문
결론
TThe nitride symbol is more than just a symbol; it encodes chemical, structural, and functional information crucial for industrial and research applications. From thermal management in AlN to semiconducting GaN and structural Si₃N₄, nitrides play a pivotal role across industries.
Understanding nitride symbols, charges, and valency allows engineers and procurement professionals to select, handle, and implement nitrides effectively. As research advances in hydrogen storage, ternary nitrides, and 2D nitrides, mastery of the nitride symbol and its chemistry will continue to unlock innovative applications.